Exhibit 10.3
INIHIBIKASE THERAPEUTICS
COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT
THIS COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT (“Agreement”) is entered into with an effective date as of the 29th day of February 2012, by and among, on the one hand, Inhibikase Therapeutics, Inc., a Delaware corporation, with offices located at 3350 Riverwood Parkway, Suite 1927, Atlanta, Georgia (the “Company”) and, on the other hand, Sphaera Pharma Pte. Ltd., a company incorporated under the laws of Singapore with its registered office at 8 Temasek Boulevard, #22-03 Suntec Tower 3, Singapore 038988 (“Sphaera Singapore”) and Sphaera Pharma Pvt. Ltd., Plot No. 32, Sector 5, IMT Manesar Haryana 122051, India (“Sphaera India”)(together with Sphaera Singapore, hereinafter referred to as “Sphaera Pharma”). (Company and Sphaera Pharma shall be referred to individually as a “Party” and collectively as the “Parties.”)
RECITALS
WHEREAS, Sphaera Pharma is an integrated drug discovery and development organization
WHEREAS, Company controls certain technology for use in the prevention, diagnosis, treatment or control of human and animal infectious diseases, particularly relating to the use of a drug approved for human use to block infection by certain bacterial and viral pathogens; and
WHEREAS, the Parties have determined that the provision of analysis and testing services offered by Sphaera Pharma is of mutual interest and benefit;
NOW THEREFORE, in consideration of the mutual covenants and promises herein, the receipt and sufficiency of which are hereby acknowledged, Sphaera Pharma and Company agree as follows:
1. Analysis and Testing Project.
a. The Scope of Work. During the Term (as defined below) of this Agreement, Company hereby engages Sphaera Pharma and Sphaera Pharma hereby agrees to perform on behalf of Company various analysis, research and testing services (the “Services”) as are described on that certain attachment entitled “Scope of Work” (the “SOW” or “Project”), which is attached hereto, made a part hereof and marked as Attachment “A.” Sphaera Pharma shall during the Term promote the interests of Company and perform the Services timely, faithfully, honestly, diligently, efficiently and professionally. Without limiting the generality of the foregoing, Sphaera Pharma hereby agrees that the Services shall be performed solely and exclusively by it.
| Page 1 |
b. Limitation on Services. In the performance of the Services, Sphaera Pharma shall (i) use those facilities, equipment, supplies and materials as are necessary to and solely and exclusively owned by it or provided to it by Company for use in the Project (the “Materials”); and (ii) engage those of its employees, including the Project Coordinator (as defined below), whose services are required for the Project on a “need to know” basis, in which he or she shall have (1) assigned to Sphaera Pharma any and all of his or her rights in intellectual property created or invented during his or her term of Sphaera Pharma employment and (2) agreed to such other terms and conditions regarding the Services, the Project Improvements and Project Results (as defined below) as are substantially similar to the terms and conditions of this Agreement, including, without limitation, the provisions of Sections 4, 5 and 6 hereof (the “Project Personnel”).
c. No Conflicting Obligation. Sphaera Pharma represents and warrants to Company on the Effective Date and on each day of the Term that its performance of the Services and all other terms and conditions of this Agreement and as a consultant to Company does not and will not breach any agreement between it and any other Person. Sphaera Pharma has not entered into, and agrees it will not enter into, any agreement, either written or oral, that is or shall be in conflict with this Agreement.
d. Pre-Existing Property. The Parties shall identify in the SOW any and all Pre-Existing Property (as defined below) that may be necessary or useful to the Project. For purposes of this Agreement, (i) “Pre-Existing Property” shall mean either Pre-Existing Intellectual Property (as defined below) or Materials (or both); (ii) “Pre-Existing Intellectual Property” shall mean any and all intellectual property, data or information created, developed, conceived or invented, whether or not reduced to practice, that is owned or in which rights are held by the Provider; (iii) “Provider” shall mean the Party who owns or has rights in or is deemed to own or have rights in any and all such Pre-Existing Property, the Project Results or Project Improvements (as such terms and phrases are defined in this Agreement) that is delivered or otherwise made available to the Recipient; and (iv) “Recipient” shall mean the Party who is in receipt of any such property.
e. Materials. All Materials made or to be made available for use in a Project shall be described in the SOW, which description shall include Provider’s name and the name or nature, amount or volume, source or origin and any and all restrictions, whether contractual, legal or otherwise, on the use of the Materials.
2. Consideration for Services.
a. The Project Fees. For and in consideration for the Services, Company shall pay to Sphaera Singapore (for and on account of the Services to be performed by either or both of Sphaera Singapore and Sphaera India) the Project Fixed Fees, Project Variable Fees, Project Milestone Fees and Project Percentage Fees (as each are defined below)(together, the “Project Fees”) in accordance with the following terms and conditions:
i. Project Fixed Fee. A fixed fee for the Services as mutually defined in the SOW(s)(the “Project Fixed Fee”);
ii. Project Variable Fee. A variable fee for the Service in the mutually agreed amount proportionate to the effort put into any additional work defined by a Change Orders to the SOW.
| Page 2 |
iii. Project Milestone Fees. In addition to the Project Fixed Fees, milestones are to be paid in the following amounts, the payment of which is contingent upon achievement of each of the following milestones (the “Project Milestone Fees”):
| Milestone Event | Payment | |||
| First dosing of patient in US Phase 1 trial | $ | 250,000 | ||
| US Phase 1 trial completion with endpoints met | $ | 500,000 | ||
| US Phase 2 trial completion with endpoints met | $ | 875,000 | ||
| FDA Approval | $ | 4,000,000 | ||
iv Project Percentage Fees. A project percentage fees payment equal to a percentage of annual net sales, if any, from the sale of the new chemical entity described in the SOW (the “NCE”) to an end user by Company or any sublicensee thereof during that period beginning with the first commercial sale and ending on the earlier to occur of either the fifteenth (15th) anniversary of such sale or the expiration of the first patent in which claims covering the NCE is issued in the United States (the “Project Percentage Fee”):
| Rate | Amount of Annual Net Sales | |
| 7% | For that portion of annual net sales that are less than or equal to $500 million | |
| 5% | For that portion of annual net sales that are greater than $500 million |
b. Sphaera Singapore As Designated Representative. Sphaera Singapore is hereby irrevocably appointed as representative, agent and attorney-in-fact for each of Sphaera Singapore and Sphaera India (i) to give and receive notices and communications relating to the transactions and other matters contemplated by this Agreement, including those relating to the payment of the Project Fees and indemnification claims; (ii) to make decisions on behalf of each of Sphaera Singapore and Sphaera India with respect to the transactions and other matters contemplated by this Agreement, and (iii) to take other actions on behalf of the Sphaera Singapore or Sphaera India (or both) as contemplated by this Agreement, including the exercise of all rights granted to the Sphaera Singapore or Sphaera India (or both) under this Agreement. Each of Sphaera Singapore and Sphaera India agree that Company may rely conclusively on the written instructions or notices delivered to Company by the Sphaera Singapore.
c. Payment Due Dates. Company will make the Project Milestone Fee payments to Sphaera Singapore for the Services no later than sixty (60) days after the achievement by Company of the applicable conditions or milestones as are set forth above. Payment for Project Fixed Fee and Project Variable Fee will occur as described in the SOW.
| Page 3 |
d. Interest. In the event that any payment due hereunder is not made when due, any such undisputed payment shall accrue interest beginning on the first day following the calendar month to which such payment relates, calculated at a annual rate equal to 3%. Such payment when made shall be accompanied by all interest so accrued. Said interest and the payment and acceptance thereof shall not negate or waive the right of Sphaera Pharma to any other remedy, legal or equitable, to which it may be entitled because of the delinquency of the payment.
e. Audit of Records. Company shall maintain complete and accurate records sufficient to enable accurate calculation of the Project Fees due to Sphaera Pharma under this Agreement. Once a calendar year for the period during which Company is obligated to pay the Project Fees, Sphaera Pharma shall have the right to select a certified public accountant to inspect, on reasonable notice and during regular business hours, Company’s records to verify its statements and such payments due pursuant to this Agreement. The entire cost for such inspection shall be borne by Sphaera Pharma, unless there is a discrepancy of under-reporting or underpayment greater than 10% in any twelve (12) consecutive calendar month period, in which case Company shall bear the entire cost of the inspection, as well as any additional sum that would have been payable to Sphaera Pharma had Company reported correctly, plus interest thereon.
3. Project Conferences, Reports and Plan Modifications.
a. Project Conferences. During the Term (as defined below), Sphaera Pharma shall cause its Project Personnel to meet with Company to discuss and evaluate the progress of the Project at such times, no less often than at the times designated in the SOW or, if not designated, then monthly and at the termination or expiration of this Agreement. Such meetings may be held virtually, using video conference, or in person at such other locations as may be mutually agreed. Consistent with the foregoing, Sphaera Pharma shall provide Company with (i) written progress reports on the Project no less frequently than as shall be provided in the SOW and, if not provided, then weekly, and (ii) a final written report of the Project submitted to Company no later than forty-five (45) days after completion of the Project or the termination or expiration of this Agreement, whichever event first occurs (collectively the “Project Reports”).
b. Facility Visits. Upon reasonable advance notice, each of Sphaera Singapore and Sphaera India shall permit Company representatives to visit its respective facilities during normal working hours and with reasonable frequency, to observe Project progress, discuss the Project with appropriate Project Personnel and inspect and copy records and data relevant to the Project. Facility visits by Company shall also be permitted during the records and data retention period described in this Section below. During facility visits, Company may inspect, but shall not be permitted to copy or remove, in whole or in part, any of Sphaera Pharma’s standard operating procedures (SOPs).
c. Project Reports and Records. In each Project Report, the Project Coordinator shall describe (i) the Project Results and any and all of the Services performed by Sphaera Pharma in accordance with the Statement of Work; and (ii) any and all Project Improvements. Any and all records relating to the Project shall be maintained by Sphaera Pharma for a period of five (5) years following the last day of the Term or for such longer period as may be required by any regulatory authority having jurisdiction over the sale of the NCE.
| Page 4 |
d. Modification of SOW. Should Company want to change a SOW or to include additional Services to be provided by Sphaera Pharma, Company shall propose to Sphaera Pharma such change or other modification in a written amendment thereto (a "Change Order"). If Sphaera Pharma agrees to such Change Order, Sphaera Pharma will evidence its agreement to such Change Order by countersigning the same. The SOW as modified by such Change Order shall be binding on the Parties only if signed by all Parties, whereafter such modified version of the SOW will be deemed to have amended and replaced the prior version thereof.
4. Intellectual Property Rights.
a. Ownership. As between the Parties, Company shall own all right, title and interest in and to Company’s Pre-Existing Property, Project Results and the Project Improvements. Sphaera Pharma will own right, title and interest in and to Sphaera Pharma’s Pre-existing Property.
b. Definitions. For purposes of this Agreement, the following terms and phrases shall have the meaning ascribed thereto:
(i) “Project Improvements” shall mean any Intellectual Property conceived or reduced to practice under this Agreement or within scope of the SOW made a part hereof by one or more employees of Sphaera Pharma that results from or constitutes improvements in or additions to the Company’s Pre-Existing Property, including, but not limited to, any know-how, inventions, designs, techniques, innovations or other discoveries; and
(ii) “Project Results” shall mean, without limitation, (1) any discovery, invention, innovation, development, characterization, identification or selection (including, without limitation, any and all processes, methods, assays, protocols, tests, services, treatments, targets, products, molecules, cells, proteins, peptides or nucleic acids) and any method of deriving, making, maintaining, using or manufacturing the same that either (A) is derived from, arises out of or in connection with the use of the Company’s Pre-Existing Property or performing the Services in accordance with the SOW; or (B) would not, but for the use of Company’s Pre-Existing Property or performing the Services in accordance with the SOW, have been identified, discovered or developed and rights thereto (including, without limitation, the Project Deliverables referenced in the SOW and patent applications filed in connection therewith or patents issued thereon); and (2) any progeny, replication or derivative of Company’s Material, including, without limitation, the NCE.
c. Assignment of Rights.
(i) Cooperation. Sphaera Pharma hereby assigns to Company all right, title and interest in any and all Project Improvements and Project Results. At the request of Company in the event of assignment, Sphaera Pharma shall execute such assignments, documents and other instruments as may be necessary or desirable to fully and completely assign any Project Improvements and Project Results to Company and to assist Company in applying for, obtaining and enforcing patents or copyrights or other rights with respect thereto. If Company requests, at Company's expense, Sphaera Pharma will provide Company with reasonable assistance to obtain patents covering any such Project Improvements and Project Results and convey any and all right, title and interest it may have in any such Project Improvements and Project Results to Company.
| Page 5 |
(ii) If the Company chooses to not pursue patents for Project Results that otherwise constitute jointly-owned intellectual property derived during the Term of this Agreement, Sphaera Pharma reserves the right to file on their own behalf.
(iii) If appropriate under definitions of inventorship, Company and/or its scientist(s) shall be listed as co-inventors of the Project Improvements and Project Results for their participation in the development and execution of the testing plan, procedures and related protocols.
5. Restrictions on Disclosure of Confidential Information.
a. Definition. Each of Sphaera Pharma and Company acknowledges that it may be necessary for the Provider to disclose information to the Recipient that is considered by the Provider to be its proprietary or confidential information in order for Sphaera Pharma to perform the Services relating to a proposed or actual Project. To preserve the proprietary or confidential nature of such information, Sphaera Pharma and Company agree to either: (i) clearly mark the term “CONFIDENTIAL INFORMATION” upon the information and forward it only to the Recipient in writing; or (ii) orally disclose to the Recipient the proprietary or confidential nature of the information, subsequently indicate the nature of such information contained therein and in a writing addressed to the Recipient and clearly mark the writing or information with the term “CONFIDENTIAL INFORMATION” and deliver it to the Recipient within thirty (30) days of disclosure (all such information so marked or designated being “Confidential Information”). For purposes of this Agreement, each SOW and any and all information relating thereto, including, without limitation, the Project Results and Project Improvements shall constitute, as between the Parties, the proprietary and Confidential Information of Company, with Company being deemed the Provider thereof; and all Sphaera Pharma Pre-Existing Property shall constitute the Confidential Information of Sphaera Pharma. For the purposes of this agreement, the phrase “Trade Secret” shall mean information (including, but not limited to, Confidential Information) that: (y) derives economic value, actual or potential, from not being generally known to, and not being readily ascertainable by proper means by, other persons who can obtain economic value from its disclosure or use; and (z) is the subject of efforts that are reasonable under the circumstances to maintain its secrecy. To the extent that applicable law mandates a definition of "trade secret" inconsistent with the foregoing definition, then the foregoing definition shall be construed in such a manner as to be consistent with the mandated definition under applicable law.
b. Restrictions. Without the Provider’s prior written consent, Recipient shall refrain from (i) disclosing or otherwise causing to be disclosed any of Provider’s Confidential Information for a period of five (5) years from the termination of this Agreement, provided, however, that in the case of Confidential Information that constitutes a Trade Secret (as defined below), such period shall run for the period during which any such information continues to constitute a Trade Secret, and (ii) except as otherwise provided in the SOW, using any such information for any purpose whatsoever.
| Page 6 |
c. Recipient’s obligation of non-disclosure shall not apply to any or all of information that is evidenced by contemporaneously written records and:
(i) is in the public domain at the time of disclosure;
(ii) becomes part of the public domain after disclosure through no fault of Recipient;
(iii) is in Recipient’s possession at the time of disclosure or is properly obtained by Recipient from a third party with a valid legal right to disclose such information and such third party is not under a confidentiality obligation to the Provider;
(iv) has been independently developed by Recipient prior to the Effective Date; or
(v) is required to be disclosed by operation of law, governmental regulation or court order; provided, however, Recipient shall use commercially reasonable efforts to provide Provider at least 30 days’ notice prior to such disclosure. Recipient further agrees to use all reasonable effort to cooperate in securing confidential protection for such information; further, provided, that in all cases, Recipient shall limit strictly any such disclosure to the information that is requested hereunder.
d. Publicity. Company shall not use the name of Sphaera Pharma or any Project Personnel in any publicity, advertising, or news release without the prior written approval of an authorized representative of Sphaera Pharma. Sphaera Pharma shall not use the name of Company or any employee of Company in any publicity, advertising, or news release, without the prior written approval of Company.
e. Return of Materials. Upon and coincident with either the termination or expiration of this Agreement, at the election and request of Provider, Recipient agrees to either return to Provider or destroy any and all Materials and other Confidential Information, as well as permanently delete all electronically or otherwise stored Confidential Information from all systems containing such Confidential Information, and if destruction is requested, Recipient shall provide to Provider a Certificate of Destruction and Compliance in the form attached as Attachment "C."
f. Ancillary Provisions. Sections 4, 5 and 6 of this Agreement, along with the Schedules applicable thereto, shall be construed as an agreement ancillary to the other provisions of this Agreement and the existence of any claim or cause of action of Sphaera Pharma against Company, whether predicated on this Agreement or otherwise, shall not constitute a defense to the enforcement by Company of such Sections.
| Page 7 |
g. Tolling. Each Party hereby expressly acknowledges and agrees that in the event the enforceability of any of the terms of Section 6 of this Agreement shall be challenged in court or pursuant to arbitration and the other Party is not enjoined (either temporarily or permanently) from breaching any of the restraints set forth in this Agreement, then if a court of competent jurisdiction or arbitration panel finds subsequently that the challenged restraint is enforceable, the time period of the restraint shall be deemed tolled upon the filing of the lawsuit challenging the enforceability of the restraint until the dispute is finally resolved and all periods of appeal have expired.
6. Restrictions on the Use of Provider’s Property.
a. In General. As between the Parties, any and all Pre-Existing Property is and shall constitute and remain for all purposes the sole and exclusive property of the Provider.
b. Restriction on Use Provider’s Property. Except as otherwise expressly provided in this Agreement or the SOW, (i) Recipient shall limit its use of the Provider’s property (e.g., in the case of Company, Company’s Pre-Existing Property, the Project Results and Project Improvements; and, in the case of Sphaera Pharma, Sphaera Pharma’s Pre-Existing Property)(collectively, the “Project Property”) solely and exclusively to the purposes described in this Agreement and for no other purpose whatsoever; (ii) no option, license or other conveyance of rights, express or implied, is granted by Provider to Recipient or any other person, including, without limitation, any Project Personnel, in connection with any of Provider’s Project Property; (iii) none of the Provider’s Property, in whole or in part, (1) may be made or sold, licensed or otherwise transferred to a third party by Recipient; (2) will be used by Recipient in human subjects, in clinical trials, or for diagnostic purposes involving human subjects without the prior written consent of Provider; (3) is to be used by Recipient at any location other than at Recipient's laboratory or by any individual or other person other than by the Project Personnel; (4) will be used by Recipient for any purpose, other than as expressly permitted under this Agreement and in compliance with all applicable laws, and in no event for any commercial or competitive purposes. Sphaera Pharma further agrees that it shall use Company’s Pre-existing Property in the configuration in which they are received, may not under any circumstance manufacture or transform them to any other configuration and any such Services will be subject to the rights of third parties, if any, whether under license therefrom or otherwise and this Agreement and the SOW.
c. No Reverse Engineering. Sphaera Pharma hereby acknowledges that certain of Company’s Pre-existing Intellectual Property and Confidential Information provided by it to Sphaera Pharma may be encoded or otherwise “cloaked” to protect and maintain the confidentiality thereof from Sphaera Pharma and, in any such case, Sphaera Pharma agrees to refrain and shall cause each person acting for and on its behalf, including, without limitation, the Project Personnel, to refrain from engaging in any act or attempt to act by which or as a result of which any such Pre-existing Intellectual Property or Confidential Information would be reverse engineered, decompiled, translated, interpreted, decoded, revealed or otherwise identified.
| Page 8 |
d. Materials. The Materials shall be used with prudence and appropriate caution in any experimental work and in compliance with this Agreement, the applicable SOW and all applicable statutes, regulations and other applicable governmental rules, including, without limitation, the National Institutes of Health guidelines on the use of animals and recombinant DNA. The Materials may not be used for in vivo testing in human subjects. Materials derived from human donors may not be transferred with any individual donor-identifying information. Except as otherwise expressly provided in the SOW, THE MATERIALS ARE PROVIDED WITHOUT WARRANTY OF MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED.
e. No Publication. Notwithstanding any provision of this Agreement to the contrary, in no event shall Sphaera Pharma have any right to publish or otherwise use or disclose Company’s Project Property, including, without limitation, the Project Improvements and Project Results, without the prior written consent of Company, which consent may be withheld, denied, conditioned or delayed in Company’s sole and absolute discretion.
7. Indemnification.
a. Sphaera Pharma Indemnification. Sphaera Pharma shall indemnify Company and each of its affiliates and each director, officer, employee, agent, representative, successor and assign thereof (the “Company Indemnified Parties”), and defend and hold each of them harmless, from and against any and all third party claims, lawsuits, losses, damages, liabilities, penalties, costs and expenses (including reasonable attorneys’ fees and disbursements) (collectively, “Third Party Losses”) incurred by any of them in connection with, arising from or occurring as a result of (i) the material breach by Sphaera Pharma of any of any term or condition of this Agreement; (ii) Sphaera Pharma’s negligence, willful misconduct or violation of applicable law in the performance of this Agreement, and (iii) the enforcement by Company of its rights under this Section 7(a), except, in each case, for those Third Party Losses for which Company has an obligation to indemnify the Sphaera Pharma Indemnified Parties pursuant to Section 7(b), below, as to which Third Party Losses each Party shall indemnify the other Party to the extent of its respective liability for such Third Party Losses.
b. Company Indemnification. Company shall indemnify Sphaera Pharma and each of its affiliates and each director, officer, employee, agent, representative, successor and assign thereof (the “Sphaera Pharma Indemnified Parties”), and defend and hold each of them harmless, from and against any and all Third Party Losses incurred by any of them in connection with, arising from or occurring as a result of (i) the material breach by Company of any term or condition of this Agreement, (ii) any violation of applicable law in the performance of its obligations under this Agreement, and (iii) the enforcement by Sphaera Pharma of its rights under this Section 7(b), except, in each case, for those Third Party Losses for which Sphaera Pharma has an obligation to indemnify the Company Indemnified Parties pursuant to Section 7(a), as to which Third Party Losses each Party shall indemnify the other Party to the extent of its respective liability for such Third Party Losses.
8. Hazardous Materials. All Materials provided for use in a Project must be accompanied by the applicable environmental and safety information for those materials as required by law. The responsibility for and costs of disposal of all Provider Materials remaining at the termination of the SOW will rest with the Provider. Provider shall arrange for disposal or removal of any remaining Provider Materials prior to receipt of any final report of the Project. Sphaera Pharma will observe all applicable safety precautions and governmental requirements concerning handling of test materials.
| Page 9 |
9. Independent Contractor. For the purposes of this Agreement and all services to be provided hereunder, the Parties shall be, and shall be deemed to be, independent contractors and not agents or employees of the other Party. Neither Party shall have the authority to make any statements, representations or commitments of any kind or to take any action which shall be binding on the other Party, except as may be expressly provided herein or authorized in writing.
10. Re-purchase Option and Development Right re Field of Cancer.
a. Abandonment. Notwithstanding anything set forth in this Agreement, should Company or any successor-in-interest thereof decide in its sole discretion to abandon the development or commercialization of the NCE that results from the SOW, Company or its successor-in-interest shall give written notice thereof to Sphaera Pharma (the “Abandonment Notice”). On the thirtieth (30th) Business Day following the day that the Abandonment Notice was delivered to Sphaera Pharma (the “Repurchase Period”), Sphaera Pharma shall have the irrevocable right and option to acquire, and upon due exercise of such option, Company or any successor thereof shall sell to Sphaera Pharma, the NCE to the extent of its unencumbered rights therein and controlled by Company or such successor.
b. Repurchase Notice. Sphaera Pharma may exercise its right to purchase the NCE by delivering written notice of the same to Company or successor thereof at any time during the Repurchase Period (“Repurchase Notice”). Should Sphaera Pharma fail to deliver such written notice to Company or such successor during the Repurchase Period, the rights of the Sphaera Pharma shall be null and void.
c. Purchase Price. The purchase price for the NCE shall be an amount equal to the NCEs fair market value at the point in development it has been taken by Company. The closing of the purchase and sale of NCE shall occur within thirty (30) days following the delivery of Repurchase Notice, or such other time as Company or such successor and Sphaera Pharma shall mutually agree.
d. Condition to Sale. The obligation of Company or any successor thereof to sell the NCE under this Section to Sphaera Pharma shall be conditioned on Sphaera Pharma otherwise being in compliance with the terms of this Agreement and delivering at closing a full and complete general release of any and all claims it may have against Company or any successor thereof and the securing of any and all necessary third party consent.
e. Development Right. Inhibikase hereby agrees that Sphaera Singapore will have the right to develop the NCE for use in the treatment of cancer in humans; provided, however, that Sphaera Pharma shall use commercially reasonable efforts in any such development efforts, undertake any and all such development activities in compliance with this agreement and applicable standards, guidelines, regulations and laws, and indemnify and hold harmless Company from any and all damages Company may incur as a result thereof.
| Page 10 |
11. Notice of IND Enabling Studies. If Company determines to conduct IND enabling studies, it agrees to notify Sphaera Pharma of such determination, at which time the parties will discuss whether Sphaera can assist in the advancement of the SOW work product into clinic and, if so, at what cost.
12. Term and Termination.
a. Term. The term of this Agreement shall commence with Effective Date and terminate upon that date which coincides with the last day of the Term (as defined below) (such date shall be referred to as the “Expiration Date”); provided, however, that in no event shall the expiration of this Agreement occur prior to the date on which the obligations by one Party to the other Party shall have lapsed under any SOW (together, the "Term"). For purposes of this Agreement, the phrase “Term” shall mean that period from the Effective Date through and including the one hundred and eightieth (180) day thereafter.
b. Termination For Cause. Upon any material breach of this Agreement by a Party (the “Breaching Party”), the other Party (the “Non-Breaching Party”) may terminate this Agreement by providing ninety (90) days' written notice to the Breaching Party of the occurrence and nature of such material breach. The termination shall become effective at the end of the notice period unless the Breaching Party cures such breach during the notice period. The Non-Breaching Party may, by notice to the Breaching Party, designate a later date for such termination in order to facilitate an orderly transition of activities relating to the Product or Process. Notwithstanding the foregoing, if such breach, by its nature, is curable, but not within the forgoing cure period, then such cure period shall be extended if the Breaching Party provides a written plan for curing such breach to the Non-Breaching Party and uses diligent efforts to cure such breach in accordance with such written plan; provided, however, that no such extension shall exceed one-hundred twenty (120) days without the consent of the Non-Breaching Party; and in the event of a dispute as to whether performance has been made by either Party pursuant to this Agreement, the relevant cure period with respect thereto shall be tolled pending resolution of such dispute in accordance with the applicable provisions of this Agreement.
c. Accrued Rights. Termination or cancellation of this Agreement shall not effect the rights and obligations of the parties accrued prior to termination.
d. Survival. Notwithstanding anything to the contrary, as contained herein any provision of this Agreement which by their nature extend beyond termination or expiration, shall survive such termination or expiration, including but not limited to the provisions of Section 2, 3, 4, 5, 6 and 7.
13. Notice. Any notice required by this Agreement shall be given by registered or certified mail, return receipt requested, addressed in the case of Sphaera Pharma to:
Sphaera Pharma Pte. Ltd.
8 Temasek Boulevard
#22-03 Suntec Tower 3
Singapore 038988
Attn: Dr. Frank Hollinger
| Page 11 |
| With a copy to: | Sphaera Pharma Pvt. Ltd. |
| Plot No. 32, Sector-5, | |
| IMT Manesar-122051 | |
| Atten.: Abhinav Dhandia, Manager-Corporate Affairs & Development |
or in the case of Company to:
Inhibikase Therapeutics, Inc.
3350 Riverwood Parkway
Suite 1927
Atlanta, Georgia
Attn: President
or at such other addresses as may be given from time to time in accordance with the terms of this notice provision.
14. Results of Project. Sphaera Pharma will conduct the Services in accordance with generally-accepted professional standards of workmanship and effort. NEITHER PARTY MAKES ANY WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, AND HEREBY DISCLAIMS ALL SUCH WARRANTIES AS TO ANY MATTER WHATSOEVER, INCLUDING, WITHOUT LIMITATION, WARRANTIES WITH RESPECT TO: (a) THE PROJECT AND ANY RESULTS OF THE PROJECT; (b) DATA, REPORTS, INFORMATION OR RESEARCH PROVIDED BY SPHAERA PHARMA OR COMPANY; AND (c) ANY INVENTION OR PRODUCT, OR OWNERSHIP THEREOF, WHETHER TANGIBLE OR INTANGIBLE, TESTED, CONCEIVED, DISCOVERED, OR DEVELOPED IN THE PROJECT OR IN CONNECTION WITH CONDUCTING THE PROJECT UNDER THIS AGREEMENT.
15. Export Controls. Each party acknowledges that any information or materials provided by the other under this Agreement may be subject to India and U.S. export control laws and regulations, including the International Traffic in Arms Regulations (“ITAR”, 22 CFR Chapter 1, Subchapter M, Parts 120-130), Export Administration Regulations (“EAR”, 15 CFR Chapter VII, Subchapter C, Parts 730-774), and Assistance to Foreign Atomic Energy Activities (10 CFR Part 810); each party agrees to comply with all such laws.
16. Miscellaneous.
a. Neither Party may assign or otherwise encumber this Agreement in whole or in part or any rights hereunder, without the prior written consent of the other Party, such consent not to be unreasonably withheld, delayed, conditioned or denied; provided, however, that (a) Company may assign this Agreement in whole or in part to an affiliate thereof on the condition that Company shall remain liable hereunder for the prompt payment and performance of all obligations thereof, and to a third party in connection with a sale or transfer by operation of law of all or substantially all of its business or assets; provided, further, that any such assignment shall in all events be conditioned on the assignee agreeing to be bound by the terms of this Agreement.
| Page 12 |
b. Unless otherwise specified, this Agreement and its Attachments embody the entire understanding between Sphaera Pharma and Company with respect to the Project, and any prior or contemporaneous representations, either oral or written, are hereby superseded. No amendments or changes to this Agreement, including, without limitation, changes to the scope of the SOW, period of performance or budget, shall be effective unless made in writing and signed by authorized representatives of the Parties.
c. During the Term and for a period of two (2) years subsequent to the termination of this Agreement, Company shall not, directly, indirectly or through any other party or means solicit employee (s) of Sphaera Pharma for employment, hiring or engagement as an independent contractor either under its own employment or in any of its subsidiaries and/or affiliates.
d. This Agreement shall be governed by and construed in accordance with the domestic laws of the State of New York, without giving effect to any choice or conflicts of law provision or rule. Each of the Parties consents to the exclusive jurisdiction of the Federal and State Courts or arbitration sitting in New York, New York, USA, in connection with any dispute arising under this Agreement and hereby waives, to the maximum extent permitted by law, any objection, including any objection based on venue or inconvenient forum, to the bringing of any such proceeding in such jurisdiction. Subject to the foregoing and except for matters in equity (e.g., injunctive relief), in the event of any dispute, claim, question, or disagreement arising from or relating to this agreement or the breach thereof, the parties hereto shall use their best efforts to settle the dispute, claim, question, or disagreement. To this effect, they shall consult and negotiate with each other in good faith and, recognizing their mutual interests, attempt to reach a just and equitable solution satisfactory to both parties. If they do not reach such solution within a period of 60 days, then, upon notice by either party to the other, all disputes, claims, questions, or differences shall be finally settled by arbitration administered by the American Arbitration Association in accordance with the provisions of its Commercial Arbitration Rules.
[Signature page follows]
| Page 13 |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives.
| Sphaera Singapore | Inhibikase Therapeutics, Inc. | |
| Sphaera Pharma Pte. Ltd. | ||
| /s/ Sundeep Dugar | /s/ Milton H. Werner | |
| Signature | Signature | |
| Sundeep Dugar | Milton H. Werner, PhD. | |
| Printed Name | Printed Name | |
| President & CEO | President & CEO | |
| Title | Title | |
| March 2, 2012 | ||
| Date | Date | |
| Sphaera India | Read and acknowledged by | |
| Sphaera Pharma Pvt. Ltd. | Project Coordinator: Sphaera Pharma | |
| /s/ Abhinav Dhandia | /s/ Frank P. Hollinger | |
| Signature | Signature | |
| Abhinav Dhandia | Frank P. Hollinger, PhD | |
| Printed Name | Printed Name | |
| Manager, Corporate Affairs & Development | Vice President | |
| Title | Title | |
| March 2, 2012 | 2 March 2012 | |
| Date | Date |
| Page 14 |
ATTACHMENT “A”
SCOPE OF WORK
Subject to the terms of that certain agreement entitled “Collaborative Research and Development Agreement” entered into by and among, on the one hand, Inhibikase Therapeutics, Inc., a Delaware corporation, with offices located at 3350 Riverwood Parkway, Suite 1927, Atlanta, Georgia (the “Company”) and, on the other hand, Sphaera Pharma Pte. Ltd., a company incorporated under the laws of Singapore with its registered office at 8 Temasek Boulevard, #22-03 Suntec Tower 3, Singapore 038988 (“Sphaera Singapore”) and Sphaera Pharma Pvt. Ltd., having registered office at E-375, First Floor, Greater Kailash-II, New Delhi-110048, INDIA (“Sphaera India”) (together with Sphaera Singapore, hereinafter referred to as “Sphaera Pharma”) (the “Collaborative Agreement”), Company hereby grants Sphaera Pharma a limited, revocable license to use Company’s Pre-Existing Property (as described below), which are to be held in trust for Company and used solely and exclusively for research and development by Sphaera Pharma in accordance with the terms of this SOW and protocols as approved by Company, which testing shall be conducted by the Project Coordinator and such other Project Personnel as may be employed by Sphaera Pharma (the “Internal Use License”). Nothing in this Agreement shall be construed to grant to Sphaera Pharma any rights in the Company Project Property, including, without limitation, the Materials, other than the Internal Use License as expressly provided in this Agreement, or to preclude Company from any use of or from granting any license for any use of the Materials.
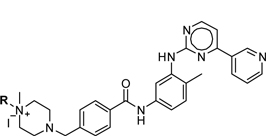
Project Overview: Modification of the Abelson tyrosine kinase inhibitor Imatinib to prepare a modified drug with a desired pharmacokinetic properties profile.
Project Personnel:
Project Coordinator: Dr. Frank P. Hollinger
Project Deliverables
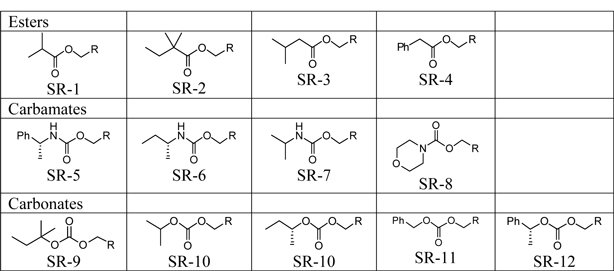
| 1) | Design and synthesize 13 – 15 modified drug analogs of Imatinib to potentially identify compounds with reduced Cmax –– and increased Cmin in mice. |
a. Evaluate compounds for solubility
b. Evaluate compounds for stability (solid and in aq. solution)
c. Evaluate compounds for conversion to active ingredient (Imatinib)
d. Evaluate compounds in mouse or rat PK to determine the PK parameters such as Cmax, Cmin – using accepted and approved practices. Use Imatinib as a reference
e. Identify two compounds with the potential for further development efforts.
| 2) | Proposed compounds subject to their ability to be synthesized: |
| Page 15 |

Table 1: Sphaera Modified Drug Reagents where R =
Project Timeline
4 -5 months
Company’s Pre-Existing Property:
Pre-Existing Intellectual Property: Mechanism of action knowledge, use of tyrosine kinase inhibitors against all receptor and non-receptor human tyrosine kinases, use of imatinib against bacterial and viral pathogens as an anti-infective agent.
Sphaera Pharma’s Pre-Existing Property:
Pre-Existing Intellectual Property:
| 1) | Modified Drug Technology Platform included in but not limited to the patent application entitled “Substituted Methylformyl Reagents & Method of using the same to modify Physicochemical and/or pharmacokinetic Properties of Compounds” (application number 1092/DEL/2010). |
| Page 16 |
Project Term: 180 consecutive calendar days
Project Reports & Milestone Events:
Progress Meetings and Reports: Quarterly
Project Completion: 120 days from the date of signing
Project Endpoints: Decrease in serum Cmax of Imatinib®, increase of Cmin in mice or rat to achieve an acceptable profile.
Project Fixed Fee: In Lieu of the services as defined above, the Company agrees to pay a Fixed Fee of US$ 160,000 payable over 4 monthly installments to commence January 1, 2012.
Project Variable Fee: No modification in the Scope of Work, or costs thereof, shall be made unless and until agreed to in writing by both the Parties.
| Page 17 |
ATTACHEMENT "B"
CERTIFICATE OF PROJECT COORDINATOR
I have read and understood the terms and conditions outlined in the Collaborative Research and Development Agreement entered into by and among, on the one hand, Inhibikase Therapeutics, Inc., a Delaware corporation, with offices located at 3350 Riverwood Parkway, Suite 1927, Atlanta, Georgia (the “Company”) and, on the other hand, Sphaera Pharma Pte. Ltd., a company incorporated under the laws of Singapore with its registered office at 8 Temasek Boulevard, #22-03 Suntec Tower 3, Singapore 038988 (“Sphaera Singapore”) and Sphaera Pharma Pvt. Ltd., with its registered offices at E-375, 1st Floor, Greater Kailash-2, New Delhi – 110048, INDIA (“Sphaera India”)(together with Sphaera Singapore, hereinafter referred to as “Sphaera Pharma”)(the “Collaborative Agreement”), and the Statement of Work and I agree to abide by them in the capacity of a Project Coordinator in receiving, using and making a disclosure, if any, of the Material, the Pre-Existing Intellectual Property, Project Results and Project Improvements, including, without limitation, Company’s Confidential Information and Trade Secrets and any other intellectual property or other tangible property relating thereto. Except as otherwise defined herein, capitalized terms and phrases shall have the meaning ascribed thereto in the Collaborative Agreement.
| PROJECT COORDINATOR | |
| /s/ Frank P. Hollinger | |
|
By: Frank P. Hollinger |
| Page 18 |
ATTACHEMENT "C"
(TO BE RETYPED ON LICENSEE'S STATIONERY)
CERTIFICATE OF DESTRUCTION AND COMPLIANCE
By signing below, I hereby affirm on behalf of Sphaera Pharma, that:
All Material sent to Sphaera Pharma by Inhibikase Therapeutics, Inc. (the “Company”) ("Company") and all Project Property made pursuant to that certain Collaborative Research and Development Agreement and the related SOW, entered into by and among, on the one hand, Inhibikase Therapeutics, Inc., a Delaware corporation, with offices located at 3350 Riverwood Parkway, Suite 1927, Atlanta, Georgia (the “Company”) and, on the other hand, Sphaera Pharma Pte. Ltd., a company incorporated under the laws of Singapore with its registered office at 8 Temasek Boulevard, #22-03 Suntec Tower 3, Singapore 038988 (“Sphaera Singapore”) and Sphaera Pharma Pvt. Ltd., having registered office at E-375, First Floor, Greater Kailash-II, New Delhi-110048, India (“Sphaera India”)(together with Sphaera Singapore, hereinafter referred to as “Sphaera Pharma”)(the “Agreement”), have been returned to Company or, at Company’s prior written request, destroyed in accordance with Company's instructions.
Sphaera Pharma holds no Company Project Property at the present time, including, without limitation, the Pre-Existing Intellectual Property and Materials; and
All Project Property sent to Sphaera Pharma by Company pursuant to the Agreement to which this Attachment is attached have been used by Sphaera Pharma in full compliance with the terms and conditions the Agreement, and all work or cooperation contemplated by the SOW has been completed or terminated, and Sphaera has no further rights thereunder.
Except as otherwise defined herein, capitalized terms and phrases shall have the meaning ascribed thereto in the Agreement.
| Sphaera Singapore |
| Sphaera Pharma Pte. Ltd. |
| By: | ||
| Name: | ||
| Title: | ||
| Date: |
| Page 19 |
| Sphaera India | |
| Sphaera Pharma Pvt. Ltd. |
| By: | ||
| Name: | ||
| Title: | ||
| Date: |
| Page 20 |